Over the past 30 years the vaccine schedule for infants has greatly increased. Babies born in 2017 can receive as many as 36 doses of 14 vaccines by 15 months of age starting with the Hepatitis B vaccine on the day of birth.
Untested schedule, knowledge severely lacking
The quantity and combination of vaccines in this schedule have never been tested. The infant vaccine schedule is one size fits all. Babies’ immune systems are not tested and family histories of autoimmune or allergic conditions are not considered prior to vaccinating.
This started with the first round of childhood vaccines given in the late 1960s, as reported on page 8 of the Merck MMR II package insert:
Routine administration of DTP (diphtheria, tetanus, pertussis) and/or OPV (oral poliovirus vaccine) concurrently with measles, mumps and rubella vaccines is not recommended because there are limited data relating to the simultaneous administration of these antigens.
The CDC failed to take advantage of significant changes in vaccine formulation to justify testing of the schedule, even though these changes were specifically made due to serious safety concerns:
- when the combination MMR vaccine was introduced in 1971
- when the reactogenic smallpox vaccine was discontinued in 1972
- when the blood-derived hepatitis vaccine was discontinued in 1986
- when the acellular pertussis replaced the whole cell pertussis vaccine in 1997
- when the rhesus rotavirus vaccine was pulled from the market in 1999
- when inactivated polio replaced live poliovirus vaccine in 2000
And the CDC failed to conduct testing with the subsequent addition of many dozens of doses of nine additional vaccines added to the schedule: Hepatitis B, Hib, Varicella (chickenpox), Rotavirus, Hepatitis A, Pneumococcal (Prevnar 13), Influenza, Meningococcal, Human papillomavirus (Gardasil quadrivalent types 6, 11, 16, 18).
The infant vaccine schedule is one size fits all and the dosage does not vary. A baby born 15 weeks premature at 21 weeks gestation is given the same dosage as a postterm baby born 42 weeks or later, as per guidance from the American Academy of Pediatrics:
Parents may think that their newborns are just too fragile to be vaccinated because of low birth weight and possible health problems that came with their baby’s preterm birth. Your pediatrician will tell you that all of these babies should be given the routinely recommended childhood vaccinations. They should get every immunization on the standard schedule when they reach the ages at which these shots are normally given to all children.
We examine each of the vaccines recommended for infants from birth to 15 months of age, and the effects of each vaccine on the infant immune system. We present studies where infant immune response, side effects, vaccine efficacy, serotype replacement and duration of immunity are discussed. Studies discussing the effect of breastfeeding and maternal antibodies on the infant immune response are also presented.
The 2014 study below reveals that knowledge of vaccination’s effect on the infant immune system is severely lacking and argues “for an improved understanding of the immune responses of neonates, infants and young children to vaccine antigens.”
Challenges in Vaccination of Neonates, Infants and Young Children? (full text) Vaccine. 2014 Jun 30; 32(31): 3886–3894.
“Maternal antibody and poor generation of T-cell and B-cell memory in neonates and infants are known to result in inadequate adaptive immunity from vaccinating this population compared to older children and adults. Our group has recently identified a subset of infants and young children that fail to generate protective antibody levels to diphtheria (DT), tetanus (TT), pertussis (PT) toxoid, pertussis filamentous hemagglutinin (FHA), and pertussis pertactin (PRN) in DTaP vaccinations, polio serotype 3, and Streptococcus pneumoniae conjugated polysaccharide 23F (Prevnar-CRM) and produce lower geometric mean titers to polio serotypes 1 and 2 and, Streptococcus pneumoniae serotype 14.” … “Although challenging because of the age of the subjects, more studies to further understand immune dysfunction in neonates, infants and young children are needed. The quality and quantity of systemic and mucosal antibody and memory B-cell generation, adaptive CD4 T-cell vaccine-specific responses and memory recall, and APC – B-cell and CD4 T-cell interactions following recommended vaccinations needs to be more thoroughly characterized.”
Immune activation by aluminum adjuvants
The newborn immune system is not fully developed at birth. Immune system activation caused by vaccines and aluminum (contained in some infant vaccines) has been connected with brain injury and autism. In addition to the two studies below, see this series for further information about the effect of Immune Activation and Autism.
Do aluminum vaccine adjuvants contribute to the rising prevalence of autism? Journal of Inorganic Biochemistry Volume 105, Issue 11, November 2011, Pages 1489–1499
“Aluminum (Al), the most commonly used vaccine adjuvant, is a demonstrated neurotoxin and a strong immune stimulator. Hence, adjuvant Al has the potential to induce neuroimmune disorders. When assessing adjuvant toxicity in children, two key points ought to be considered: (i) children should not be viewed as “small adults” as their unique physiology makes them much more vulnerable to toxic insults; and (ii) if exposure to Al from only few vaccines can lead to cognitive impairment and autoimmunity in adults, is it unreasonable to question whether the current pediatric schedules, often containing 18 Al adjuvanted vaccines, are safe for children? … The application of the Hill’s criteria to these data indicates that the correlation between Al in vaccines and ASD may be causal.”
Non-linear dose-response of aluminium hydroxide adjuvant particles: Selective low dose neurotoxicity Toxicology Volume 375, 15 January 2017, Pages 48–57
“Aluminium (Al) oxyhydroxide (Alhydrogel®), the main adjuvant licensed for human and animal vaccines, consists of primary nanoparticles that spontaneously agglomerate. Concerns about its safety emerged following recognition of its unexpectedly long-lasting biopersistence within immune cells in some individuals, and reports of chronic fatigue syndrome, cognitive dysfunction, myalgia, dysautonomia and autoimmune/inflammatory features temporally linked to multiple Al-containing vaccine administrations. Mouse experiments have documented its capture and slow transportation by monocyte-lineage cells from the injected muscle to lymphoid organs and eventually the brain. … We conclude that Alhydrogel® injected at low dose in mouse muscle may selectively induce long-term Al cerebral accumulation and neurotoxic effects.”
CDC recommended vaccination schedule
Below is the CDC’s recommended childhood vaccination schedule for children from birth to 15 months. Check individual state requirements, as they differ by state.
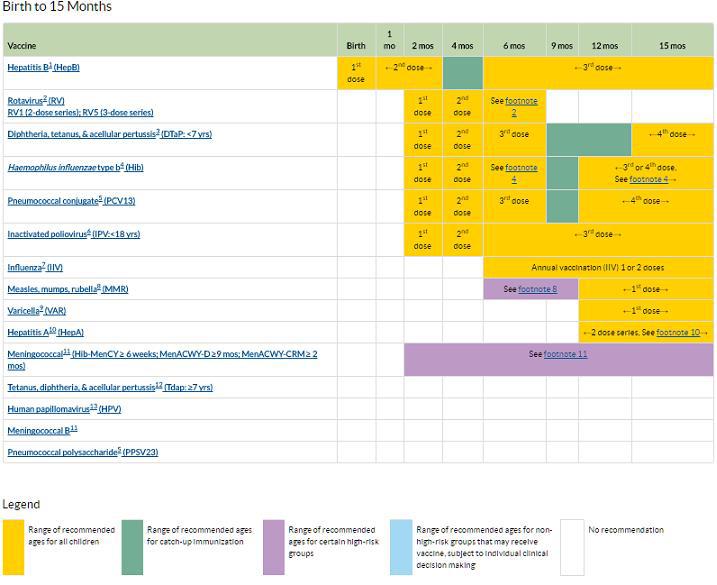 Footnotes available here
Footnotes available here
FDA Vaccines Licensed for Use in US
Hepatitis B Vaccine
The three dose series begins at birth. The genetically engineered recombinant hepatitis B vaccine was licensed in 1986, replacing the plasma derived vaccine approved in 1981. A thimerosal free vaccine was approved in 1999 after the American Academy of Pediatrics (AAP) and the U.S. Public Health Service (PHS) jointly recommended reducing infant exposure to thimerosal.
There are many studies questioning the efficacy and safety of the hepatitis b vaccine. Two recent studies report on various serious adverse events:
Statistical and Ontological Analysis of Adverse Events Associated with Monovalent and Combination Vaccines against Hepatitis A and B Diseases (full text) Scientific Reports – Nature 2016; 6: 34318.
“Engerix-B was associated with many nervous system AEs (e.g., demyelination, hyperreflexia, and optic neuritis), eye disorders (e.g., visual acuity reduced, double vision, and visual disturbance), and musculoskeletal or connective tissue AEs (e.g., fasciitis, fibrosis tendinous, and myofascitis) (Table 2).”
Isolated abducens nerve palsy following neonatal hepatitis B vaccination (full text) Journal of American Association for Pediatric Ophthalmology and Strabismus February 2014 Volume 18, Issue 1, Pages 75–76
“We report a case of sudden-onset abducens nerve palsy in an otherwise healthy 8-day-old boy following neonatal hepatitis B vaccination. A complete workup, including magnetic resonance imaging of the brain and orbits revealed no abnormalities. The patient recovered fully, with no recurrence of the abducens nerve palsy despite receiving the full course of the hepatitis B vaccine as well as other recommended immunizations through 18 months of age. We review the literature regarding vaccination-induced abducens nerve palsy and discuss the possible mechanisms of injury.”
Studies detailing the problem of non-responders to the hepatitis B vaccine are below:
Repeated vaccinations do not improve specific immune defenses against Hepatitis B in non-responder health care workers Vaccine Volume 32, Issue 51, 5 December 2014, Pages 6902–6910
“We found that the great majority of the non-responders had a functional immune system and a preserved ability to respond to other conventional antigens. Our most important findings are that the frequency of HBsAg-specific memory B cells is comparable in non-responders and controls and that booster immunization does not lead either to antibody production or memory B cell increase in non-responders.”
Celiac Disease is an autoimmune disorder which is also increasing in children.
Failure to Respond to Hepatitis B Vaccine in Children with Celiac Disease Journal of Pediatric Gastroenterology & Nutrition April 2007 – Volume 44 – Issue 4
“Conclusions: More than 50% of children with CD do not show a response to standard vaccination regimens for HBV. Given the large number of children with CD throughout the world, this observation suggests that there is a large HBV-susceptible population despite widespread vaccination. Current immunization strategies may need to be reassessed to protect this population and achieve the goal of universal protection.”
Boys and girls can respond differently to the hepatitis B vaccine, with concerns for autoimmunity in females:
HLA-DPB1 and anti-HBs titer kinetics in hepatitis B booster recipients who completed primary hepatitis B vaccination during infancy Genes and Immunity 15, 47-53 (January/February 2014)
“Gender difference was another factor found to affect the responsiveness of booster in this study. We found that female subjects had significantly stronger booster responses compared with male subjects. In the immune system, a gender gap has long been observed clinically. In humans, female subjects usually expressed higher level of antibodies and antibody-stimulating Th2 cytokines. They also had higher chances to develop autoimmune diseases because of a more responsive immune system. Our finding was consistent with previous studies, including a recent large-scale HBV vaccination cohort study.”
Systemic lupus erythematosus (SLE) has been linked to hepatitis B vaccination and the adjuvant aluminum in mice. Also concerning in this study is the vaccine and aluminum adjuvant causing brain side effects:
Immunization with hepatitis B vaccine accelerates SLE-like disease in a murine model Journal of Autoimmunity Volume 54, November 2014, Pages 21–32
“Immunization with HBVv induced acceleration of kidney disease manifested by high anti-dsDNA antibodies (p < 0.01), early onset of proteinuria (p < 0.05), histological damage and deposition of HBs antigen in the kidney. Mice immunized with HBVv and/or alum had decreased cells counts mainly of the red cell lineage (p < 0.001), memory deficits (p < 0.01), and increased activated microglia in different areas of the brain compared with mice immunized with PBS. Anxiety-like behavior was more pronounced among mice immunized with aluminum.”
Rotavirus Vaccine
Rotavirus vaccines are recommended for infants starting at 2 months of age. There are currently two live oral vaccines available in the United States: RotaTeq (approved 2006) is a pentavalent vaccine administered in a 3–dose series at ages 2, 4, and 6 months.
In 2007 the FDA issued a Public Health Notification regarding RotaTeq and intussusception (an intestinal obstruction).
Rotarix (approved 2008), is a 2–dose series given at ages 2 and 4 months. In 2012 the FDA issued an update for Rotarix that included a labelling revision regarding intussusception.
RotaShield® was licensed for use in the US in 1998 and was pulled from the market in 1999 after Reports of Intussusception.
Interference with maternal antibodies is one of the factors that can influence infant immune response to the rotavirus vaccine.
Oral Rotavirus Vaccines: How Well Will They Work Where They Are Needed Most? (full text) The Journal of Infectious Diseases (2009) 200 (Supplement_1): S39-S48.
“Of note, both interventions included a dose at 14 weeks of age; therefore, if levels of maternal antibody are a contributing factor to decreased immunogenicity, administration of a later dose when maternal titers have waned should result in improved efficacy. Although a delay in vaccination could yield a major improvement in immune response and efficacy, it would require some substantial rethinking of the routine immunization schedule currently set by the WHO immunization program at 6 and 10 weeks of age.”
Antibodies in breast milk affect the immune response of infants who receive the rotavirus vaccine, and studies recommend delaying breastfeeding to enhance the response to the vaccine in the infants.
Prevalence of rotavirus antibodies in breast milk and inhibitory effects to rotavirus vaccines (full text) Human Vaccines & Immunotherapeutics (2014) Vol. 10, Issue 12: 3681-3687
“For instance, breast feeding could be hold [sic] off at the time of vaccination and resume 1h after vaccine delivery to enhance the immune responses in children. Furthermore, this study has added to the scarce literature available on the effects of breast milk on the efficacy of RV vaccines used in different socioeconomic settings.”
Inhibitory Effect of Breast Milk on Infectivity of Live Oral Rotavirus Vaccines (full text) Pediatric Infectious Disease Journal. (2010) Oct; 29(10): 919–923.
“Some caveats should be considered in interpreting our data. First, we only examined antibody and neutralizing activity in breast milk from mothers in 4 countries; more specimens from other parts of the world including Africa and Asia should be examined to fully assess the potential negative impact of breast milk on live oral rotavirus vaccines in different settings. Second, while the observed high titers of antibody and in vitro neutralization activity of breast milk suggest a potential for substantial impact on vaccine performance, it is not possible to directly translate these findings into the real-world impact as this will be affected by many other factors such as the timing and amount of breast milk in the gut at the time of vaccination.”
This study in the journal Vaccine states that the rotavirus vaccine has had no impact on the number of infections and that vaccinated babies may not benefit.
Rotaviruses: Is their surveillance needed? Vaccine Volume 32, Issue 27, 5 June 2014, Pages 3367–3378
“Though the introduction of vaccines (RotaTeq and Rotarix) proved to be very effective in declining rotavirus associated morbidity and mortality, the number of infections remained same. Unusual genotypes significantly contribute to the rotavirus associated diarrhoeal burden, may reduce the efficacy of the vaccines in use and hence vaccinated individuals may not be benefited.”
DTaP Vaccine (diphtheria, tetanus, acellular pertussis)
DTaP was licensed for primary and booster immunization of infants and children on July 29, 1998, replacing the highly reactive DTP, licensed in 1949. It took 49 years for the dangerous DTP vaccine to be replaced, while doctors and health officials knew the pertussis portion of the vaccine was “associated with convulsions in one of 1750 doses, while severe and permanent neurologic damage has been calculated to occur with one of every 310,000 doses.”
This study from 1979 reports on “bulging fontanelle” after DTP vaccination:
Increased Intracranial Pressure After Diphtheria, Tetanus, and Pertussis Immunization (full text) American Journal of Diseases of Children 1979;133(2):217-218.
“There is a general tendency to under-report complications associated with immunizations. Part of the reason undoubtedly is related to the difficulty in proving cause and effect between the vaccine and the untoward response. However, if the complication occurs within 24 to 48 hours of administration of the vaccine in an otherwise well child, the association should arouse suspicion. We wish to report a heretofore unrecorded neurological complication, a bulging fontanelle associated with increased intracranial pressure occurring within 24 hours of a diphtheria, tetanus, and pertussis (DTP) immunization. We also wish to suggest the possibility that this complication may be missed unless specifically looked for.”
The study below examines the failure of the DTaP vaccine. Health officials can no longer blame whooping cough outbreaks on unvaccinated children.
Epidemic Pertussis and Acellular Pertussis Vaccine Failure in the 21st Century Pediatrics June 2015, VOLUME 135 / ISSUE 6
“Factors that I think are most important relating to DTaP vaccine failure are as follows: decay in antibody over time; a T helper (Th) 1/Th2 versus a Th1, Th17 cellular response; incomplete antigen package; incorrect balance of antigens in the vaccine; linked-epitope suppression; and the occurrence of pertactin-deficient Bordetella pertussis strains.4,11–18 Some, but not all, of these factors may also relate to Tdap failure over time.”
With the failure of the acellular pertussis portion of the vaccine, recently published articles are considering a return to the dangerous whole cell DTP vaccine:
The Pertussis Problem and a Possible Solution Will Parents Go Along? JAMA Pediatrics Editorial Adolescent and Young Adult Health May 2016
“What can we do to protect older children and adults from pertussis while we wait for new vaccines? The model reported by DeAngelis et al, also in this issue of JAMA Pediatrics might suggest an interim approach. This model evaluates what would happen if we brought back whole-cell pertussis vaccines. The authors suggest that we could achieve a dramatic reduction in pertussis cases by just giving an initial priming dose of whole-cell vaccine in infancy, followed by the remainder of the vaccine series with acellular vaccine.”…”But, can we really bring back whole-cell pertussis vaccine? Will parents accept a vaccine with more adverse effects? The reason acellular pertussis vaccines were developed in the first place was because whole-cell vaccine was felt by many to have unacceptably high rate of adverse effects.”
The following studies describe the shocking lack of knowledge regarding immune response to DTaP and pertussis vaccination:
Substantial gaps in knowledge of Bordetella pertussis antibody and T cell epitopes relevant for natural immunity and vaccine efficacy Human Immunology Volume 75, Issue 5, May 2014, Pages 440–451
“The recent increase in whooping cough in vaccinated populations has been attributed to waning immunity associated with the acellular vaccine.”…”Moreover, there are a limited number of studies defining epitopes from natural infection versus whole cell or acellular/subunit vaccines. The relationship between epitope location and structural features, as well as antigenic drift (SNP analysis) was also investigated. We conclude that the cumulative data is yet insufficient to address many fundamental questions related to vaccine failure and this underscores the need for further investigation of B. pertussis immunity at the molecular level.”
Functional deficits of pertussis-specific CD4+ T Cells in Infants Compared to Adults Following DTaP Vaccination Clinical & Experimental Immunology Volume 169, Issue 3 September 2012 Pages 281–291
“Understanding the immune responses that explain why infants require multiple doses of pertussis vaccine to achieve protection against infection is a high priority.“ …”Moreover, a significantly higher percentage of infant’s functional CD4+ T cells were restricted to CD45RA-CCR7+CD27+ phenotype, consistent with early stage differentiated pertussis-specific memory CD4+ T cells. We show for the first time that DTaP vaccination induced CD4+ T cells in infants are functionally and phenotypically dissimilar from those of adults.”
Earlier infantile immune maturation is related to higher DTP vaccine responses in children (full text) ClinIcal and Translational Immunology. 2016 Mar; 5(3): e65. Published online 2016 Mar 11
“Vaccination of infants and young children is required to prevent infectious diseases in early life, but its effectiveness might be impeded by immaturity of the immune system. However, longitudinal studies investigating associations between the development/maturation progress of the adaptive immune system and magnitudes of DTP vaccine-induced antibody responses in children are lacking.”
Haemophilus Influenza type B Vaccine (Hib)
The Haemophilus influenza type B (Hib) vaccine is recommended starting at 2 months of age and is available in single dose PedvaxHIB, Hiberix, Act-Hib, combined vaccines Comvax (with Hepatitis B), MenHibrix (with Men C & Y), and the 5 in 1 Pentacel (with DTaP-IPV) in a 3 or 4 dose series.
The original polysaccharide Hib vaccines introduced in the US in 1985 were not effective in the undeveloped infant immune system. To circumvent this problem, vaccine researchers developed various Hib polysaccharide-conjugate vaccines that were introduced to US infants beginning in 1988. By attaching the Hib polysaccharide to different protein carriers it was thought the vaccine would “trick” the undeveloped infant immune system into recognizing the Hib antibody.
Hemophilus influenzae Type B (Hib) Immunization Original German title: Zur Impfung gegen Haemophilus influenzae-B (HlB), Merkurstab 1994 47: 170-81.
“Vaccine efficacy increased considerably when the polysaccharide antigens to Hemophilus influenzae were conjugated with the protein antigens to tetanus or diphtheria toxoid. With this, the vaccines were as effective in infants as in older children (review in Meyer and Gahr). This trick used in vaccine production must, however, be seen as a form of ‘poisoning.’ It enables infants to develop the specific immunity which otherwise is reserved for a later time in life. It is not yet known if this premature development has disadvantages in other areas. Nor have potential consequences for the nonspecific immune system been established so far.”
JAMA published a study in 1992 indicating the new, second generation infant conjugate vaccines were worrisome and advised caution.
Impact of Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine on responses to concurrently administered diphtheria-tetanus-pertussis vaccine. JAMA 1992 Feb 5;267(5):673-8
“Concurrent administration of PRP-T [Hib Tetanus Conjugate] vaccine with DTP vaccine, either in the same syringe or at different sites, interfered with antipertussis responses to a primary series of immunizations. Although the clinical significance of this antagonism is uncertain, these data underscore the caution required in decisions to add new vaccines to existing immunization regimens.”
Important Hib vaccine concerns include autism and diabetes:
Hypothesis: Conjugate vaccines may predispose children to autism spectrum disorders Medical Hypothesis December 2011 Volume 77, Issue 6, Pages 940–947
“Conjugate vaccines fundamentally change the manner in which the immune systems of infants and young children function by deviating their immune responses to the targeted carbohydrate antigens from a state of hypo-responsiveness to a robust B2 B cell mediated response. This period of hypo-responsiveness to carbohydrate antigens coincides with the intense myelination process in infants and young children, and conjugate vaccines may have disrupted evolutionary forces that favored early brain development over the need to protect infants and young children from capsular bacteria.”
Clustering of cases of insulin dependent diabetes (IDDM) occurring three years after hemophilus influenza B (HiB) immunization support causalrelationship between immunization and IDDM Autoimmunity 2002 Jul;35(4):247-53
“Conclusion: Exposure to HiB immunization is associated with an increased risk of IDDM.”
A 2015 study revealed disturbing numbers of reactions and deaths after Hib vaccines as reported to the US VAERS reporting system.
Adverse Events following Haemophilus influenzae Type b Vaccines in the Vaccine Adverse Event Reporting System, 1990-2013 The Journal of Pediatrics April 2015 Volume 166, Issue 4, Pages 992–997
“VAERS received 29 747 reports after Hib vaccines; 5179 (17%) were serious, including 896 reports of deaths. Median age was 6 months (range 0-1022 months). Sudden infant death syndrome was the stated cause of death in 384 (51%) of 749 death reports with autopsy/death certificate records. The most common non-death serious AE categories were neurologic (80; 37%), other noninfectious (46; 22%) (comprising mainly constitutional signs and symptoms); and gastrointestinal (39; 18%) conditions. No new safety concerns were identified after clinical review of reports of AEs that exceeded the data mining statistical threshold.”
Since the introduction of Hib vaccines, non type b serotypes “associated with significant morbidity and mortality” have replaced the vaccine strain.
Invasive infections caused by haemophilus influenzae serotypes in twelve Canadian IMPACT centers, 1996-2001 Pediatric Infectious Disease Journal 2007 Nov;26(11):1025-31.
“Haemophilus influenzae type b (Hib) immunization has changed the epidemiology of pediatric bacterial invasive disease. … In 1996–2001, two-thirds of H. influenzae invasive disease in the 12 IMPACT centers was caused by non-b serotypes, which were associated with significant morbidity and mortality.”
Serious questions regarding proper doses and immune response interference in combination vaccines were reported in 2010:
Foresight in medicine: current challenges with Haemophilus influenzae type b conjugate vaccines (full text) Journal of Internal Medicine Volume 267, Issue 3 March 2010 Pages 241–250
“Additional information is however still needed e.g. about new formulations and combinations of the vaccine… The amount of antigen was determined to a large extent on the basis of the first clinical results: … it has become apparent that the response to fractional dosages can be as good as with the standard doses. … When Hib vaccine was administered mixed with other vaccines in infancy, one noticed soon that the vaccines interfered with each others’ immune responses. …. During last 10 years, one has also learned about the interferences between different conjugate vaccines. More research is needed for full understanding of the mechanisms and potential impact of these interferences, to design new combination vaccines and their rational use.”
Prevnar 13 Vaccine
Prevnar was introduced to the vaccine schedule Feb 17, 2000 as a 7-valent pneumococcal conjugate vaccine from Wyeth Pharmaceuticals to prevent invasive pneumococcal disease. Prevnar 7 was replaced by Prevnar 13 in 2010 as vaccine strains were replaced by non-vaccine strains. There are 92 pneumococcal serotypes, and this Lancet study warns, “there have been concerns that the non-vaccine serotypes (NVTs) could increase in prevalence and reduce the benefits of vaccination.”
Vaccine failure and vaccinated patients infected with a Prevnar 13 serotype are discussed in the following study. Serotype 19A was not available in Prevnar 7.
Vaccine Failures in Patients Properly Vaccinated with 13-Valent pneumococcal Conjugate Vaccine in Catalonia, a Region with Low Vaccination Coverage The Pediatric Infectious Disease Journal: April 2016 – Volume 35 – Issue 4 – p 460–463
“Among 84 patients with invasive pneumococcal infection, 32 had received at least one dose of PCV13. Seventeen of them had invasive pneumococcal infection produced by a PCV13 serotype. Among those, 9 patients were considered to have a PCV13 vaccine failure. Serotype 3 was isolated in 6 patients, serotype 19A in 2 and serotype 6B in 1.”
Further questions about the “uncertainty” of pneumococcal vaccines continue to be discussed:
Choosing between 7-, 10- and 13-valent pneumococcal conjugate vaccines in childhood: A review of economic evaluations (2006–2014) Vaccine Volume 33, Issue 14, 30 March 2015, Pages 1633–1658
“A more thorough exploration of uncertainty should be made in future analyses on this subject, as we lack understanding to adequately model herd and serotype replacement effects to reliably predict the population impact of PCVs. The introduction of further improved PCVs in an environment of evolving antibiotic resistance and under the continuing influence of previous PCVs implies that the complexity and data requirements for relevant analyses will further increase. Decision makers using these analyses should not just rely on an analysis from a single manufacturer.”
Effect of Pneumococcal Conjugate Vaccine on the Natural Antibodies and Antibody Responses Against Protein Antigens From Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in Children With Community-acquired Pneumonia. The Pediatric Infectious Disease Journal. 2016 Jun;35(6):683-9
“There was no difference on clinical baseline characteristics between vaccinated and unvaccinated children. Vaccinated children had significantly lower levels of antibodies against 4 of the studied pneumococcal antigens (P= 0.048 for Ply, P = 0.018 for pneumococcal surface protein A, P =0.001 for StkP and P = 0.028 for PcsB) and higher levels of antibodies against M. catarrhalis (P = 0.015). Nevertheless, the vaccination status did not significantly affect the rates of antibody responses against S. pneumoniae, H. influenzae and M. catarrhalis.”
The study below states that after 4 years following vaccination with Prevnar 7 there was no difference in IgG responses between the vaccinated and unvaccinated for most serotypes. The study also says that exposure to pneumococcus would be needed to maintain immunity, making way for Prevnar 13. Because of Prevnar 7, more dangerous serotypes appeared, especially serotype 19A.
Persistence of IgG Antibody Following Routine Infant Immunization with the 7-Valent Pneumococcal Conjugate Vaccine (full text) Pediatric Infectious Disease Journal: May 2015 – Volume 34 – Issue 5 – p e138–e142
“In 2010, PCV7 was replaced with a 13-valent product (PCV13, Pfizer) adding serotypes 1, 3, 5, 6A, 7F and 19A. Although studies have demonstrated that PCVs generate a substantial immune response, limited data exist on the longevity of the response during routine PCV use when vaccine serotype colonization is virtually eliminated and replacement colonization with nonvaccine serotypes has occurred.” …Conclusions: PCV serotype specific IgG concentrations 4 years following PCV vaccination do not persist above natural levels for most serotypes. Exposure to pneumococcus may be critical in maintaining persistent serotype specific IgG; the elimination of circulating vaccine type pneumococci by PCV may have effects on long-term immunity.”
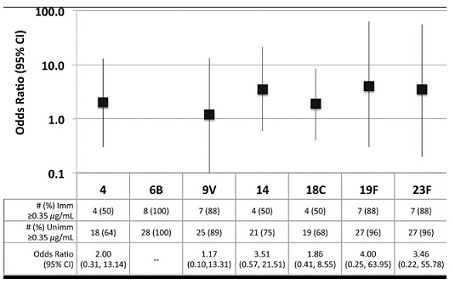
Pneumococcal vaccine failure is discussed:
Invasive Pneumococcal Disease after Routine Pneumococcal Conjugate Vaccination in Children, England and Wales (full text) CDC Emerging Infectious Diseases Volume 19, Number 1—January 2013
“Vaccine Failure PCV7-IPD occurred in 248 (20.5%) of 1,207 serotyped cases, and 52 (3.9%) of 1,332 children with IPD had 53 episodes of PCV7 vaccine failure, including 1 fully vaccinated cochlear implant recipient with 2 distinct meningitis episodes 10 months apart. Serotypes 6B (18/53 cases, 34.0%) and 19F (16/53, 30.2%) were responsible for almost two thirds of PCV7 vaccine failures. Case-patients with PCV7 vaccine failure were more likely to have comorbidities (15/52 [28.8%] vs. 166/1,155 [14.4%] case-patients with known serotype, p = 0.004). Only 1 case-patient with PCV7 vaccine failure, who had immune deficiency, died of pneumococcal meningitis 2 months after receiving a PCV7 catch-up dose in the second year of life.”
A 2003 study questions the efficacy of the pneumococcal vaccine introduced in 2000:
“Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine Volume 21, Issue 23, 4 July 2003, Pages 3265–3272
“The lack of a definitive serological correlate of protection and the multiplicity of antigens involved, especially since the clinical efficacy of most of the individual serotypes represented in the only licensed vaccine has not been established, are hindering the formulation of criteria for licensure of new formulations or combinations of the vaccine.”
Polio (Inactivated Polio Vaccine – IPV)
Inactivated polio vaccine is recommended at 2, 4, 6 and 18 months and between 4-6 years. Polio vaccines licensed in the US include IPOL (Monkey Kidney Cell) or Poliovax (Human Diploid Cell) vaccine or in the combination vaccinations Pediarix, Kinrix, Quadracel, and Pentacel. The oral polio vaccine (OPV) was discontinued in the US in the year 2000.
This study looks at VAERS reports associated with IPV vaccination (inactivated polio) from 2000-2012 with some shocking numbers:
Preparation for global introduction of inactivated poliovirus vaccine: safety evidence from the US Vaccine Adverse Event Reporting System, 2000–12 (full text) The Lancet Infectious Diseases Volume 15, No. 10, p 1175–1182, October 2015
“We classified death reports according to previously published body-system categories (respiratory, cardiovascular, neurological, gastrointestinal, other infectious, and other non-infectious) and reviewed death reports to identify the cause of death. We classified sudden infant death syndrome as a separate cause of death considering previous concerns about sudden infant syndrome after vaccines. … Of the 41,792 adverse event reports submitted, 39 568 (95%) were for children younger than 7 years. 381 of the reports for children in this age group (97%) were for simultaneous vaccination with IPV and other vaccines (most commonly pneumococcal and acellular pertussis vaccines), whereas stand alone IPV vaccines accounted for 0·5% of all reports. 34 880 reports were for non-serious events (88%), 3905 reports were for non-fatal serious events (10%), and 783 reports were death reports (2%). Injection-site erythema was the most commonly coded term for non-serious events (29%), and pyrexia for non-fatal serious events (38%). Most deaths (96%) were in children aged 12 months or younger; most (52%) had sudden infant death syndrome as the reported cause of death.” Authors have questioned the “non-specific” effects of IPV and whether it could be “detrimental for child health” and girls who received the IPV had a 52% higher mortality than boys.”
Studies have questioned the “non-specific” effects of IPV and whether it could be “detrimental for child health” and girls who received the IPV had a 52% higher mortality than boys. Also, “previous evidence suggests that IPV increases all-cause mortality by 10%.”
Is introduction of IPV “Good news for billions of children”? (full text) The Lancet Infectious Diseases Volume 16, No. 4, p409, April 2016
“In 2008, following the demonstration of methodological flaws in studies concluding that there was no sign of a negative effect of DTP, the Global Advisory Committee on Vaccine Safety stated that it would monitor the evidence of non-specific effects of vaccines. It remains to be assessed whether IPV has non-specific effects and especially whether IPV could be detrimental for child health. IPV has been used as a comparator vaccine in randomised trials; girls randomly assigned to IPV had 52% (95% 2–128) higher mortality than did boys who were randomly assigned to the vaccine.”
Changing oral vaccine to inactivated polio vaccine might increase mortality (full text) The Lancet Volume 387, No. 10023, p 1054–1055, 12 March 2016
“On average, about 75 cases of vaccine-associated paralytic poliomyelitis are reported each year worldwide, and WHO has suggested that OPV be gradually replaced by inactivated polio vaccine (IPV) to reduce the number of such cases. Results from a randomised trial in 2015 suggest that OPV might have beneficial non-specific effects that reduce all-cause mortality by 17%, possibly to a greater extent in boys than in girls, whereas previous evidence suggests that IPV increases all-cause mortality by 10%. Consequently, the proposed change from OPV to IPV might lead to increased all-cause mortality through loss of the beneficial non-specific effects of the live vaccine, and adverse non-specific effects of the inactivated vaccine. Replacement of OPV with IPV could translate to approximately 4000 deaths for each case of vaccine-associated paralytic poliomyelitis prevented, and might cause more than 300 000 additional deaths each year.”
There are variable effectiveness rates for the IPV in newborns, from as low as 8% to a high of 100%.
Oral and inactivated poliovirus vaccines in the newborn: A review Vaccine Volume 31, Issue 21, 17 May 2013, Pages 2517–2524
“There were four studies of IPV in newborns with a seroconversion rate of 8–100% for serotype 1, 15–100% for serotype 2, and 15–94% for serotype 3, measured at 4–6 weeks of life.”
Biosecurity risks in the production of IPV and polio virus strain replacement are discussed in this paper.
New Strains Intended for the Production of Inactivated Polio Vaccine at Low-Containment After Eradication (full text) PLOS Pathogens 2015 Dec; 11(12)
“Most manufacture of inactivated polio vaccines currently requires the growth of large amounts of highly virulent poliovirus, and release from a production facility after eradication could be disastrous; WHO have therefore recommended the use of the attenuated Sabin strains for production as a safer option although it is recognised that they can revert to a transmissible paralytic form. … New polio vaccines will be needed to safeguard global eradication: Sabin strains are known to evolve to fill the niche left by wild-strains so their long-term use is incompatible with eradication; most current inactivated vaccine is made from wild polioviruses so that production presents a significant biosecurity risk.”
Influenza Vaccine
The influenza vaccine is recommended beginning at 6 months of age along with a second dose given at least four weeks apart for children receiving the flu vaccine for the first time. Multi-dose vials of flu vaccine licensed for children contain the known neurotoxin “Mercury (from thimerosal)” as high as 25 µg/0.5 mL dose. The CDC does not specify that thimerosal-free vaccines be given to infants and children, even though these vaccines are available.
Pregnant women are also encouraged by the CDC to get the flu shot “at any time, during any trimester, while you are pregnant” There is no recommendation for pregnant women to receive thimerosal free vaccinations. Protection of infants is limited:
Influenza Vaccination of Pregnant Women and Protection of Their Infants The New England Journal of Medicine 2014; 371:2340 December 11, 2014
“One of the primary outcomes of the study was the efficacy of IIV3 administered during pregnancy to protect infants against confirmed influenza. The vaccine efficacy was reported to be 48.8% among infants whose mothers did not have human immunodeficiency virus (HIV) infection. This means that although their mothers were immunized, 51.2% of the infants were not protected.”
The influenza vaccine efficacy in infants is poor according to these studies:
Influenza vaccination in pediatric age (full text) Expert Review of Vaccines Volume 14, 2015 – Issue 6 Pages 785-787 | Published online: 15 Apr 2015
“The traditional inactivated influenza vaccine (IIV) – both the old formulation containing three viruses and the more recent preparation with four components – is poorly immunogenic in younger subjects; does not protect a significant number of infants, particularly when mismatched viruses are circulating; and is not licensed for those under 6 months of age. Regarding immunogenicity, younger children are quite similar to the elderly, who, because of the senescence of their immune system, respond poorly to immune stimulation”
Failure of inactivated influenza A vaccine to protect healthy children aged 6-24 months Pediatrics International 2004 Apr;46(2):122-5.
“Inactivated influenza vaccine did not reduce the attack rate of influenza A infection in 6-24 month old children.”
Vaccines for preventing influenza in healthy children Pediatrics International Volume 46, Issue 2 April 2004 Pages 122–125
“In children under the age of two, the efficacy of inactivated vaccine was similar to placebo.”
Female infants have stronger responses to influenza vaccination and “significantly increased serum levels of proinflammatory molecules” according to this PNAS study:
Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination (full text) Proceedings of the National Academy of Sciences (PNAS) vol. 111 no. 2 > 869–874, doi: 10.1073/pnas.1321060111
“In this study, we have used a systems approach to the analysis of sex differences in the immune system in humans. These data reinforce and extend previous reports, and point toward a mechanistic hypothesis that may drive the sex disparities observed in responses to vaccination. Differences in vaccine responsiveness in males versus females have been reported for most commercially available vaccines including yellow fever, influenza, measles, mumps, rubella, and hepatitis, among others. As in these studies, we find stronger responses to influenza vaccination and significantly increased serum levels of proinflammatory molecules in females compared with males, specifically LEPTIN (25), IL-RA and CRP.”
Many influenza vaccine adverse event studies are supported by vaccine manufacturers with authors having conflict of interest. Here is one example of a trial including infants:
Immunogenicity and Safety of an Inactivated Quadrivalent Influenza Vaccine Candidate: A Phase III Randomized Controlled Trial in Children (full text) The Journal of Infectious Diseases (2013) 208 (4): 544-553.
“Among children age 6–35 months in the QIV-only arm, 7 children (2.3%) reported a total of 10 SAEs. Four SAEs in 3 children were considered by the investigator to be related to the study vaccines: 2 SAEs (angioedema and acute conjunctivitis), which resolved within 7 days, were reported for a 12-year-old boy given TIV-Yam. A 1-year-old girl had a generalized seizure on the day after QIV receipt, and a 2-year-old boy had a febrile seizure 18 days after QIV receipt; both recovered within 1 day.” …”Financial support. This work was supported by GlaxoSmithKline Biologicals SA; GlaxoSmithKline SA was involved in all stages of the study conduct and analysis. GlaxoSmithKline SA also bore the costs associated with the development and the publishing of the present manuscript.”
MMR Vaccine (measles, mumps, rubella)
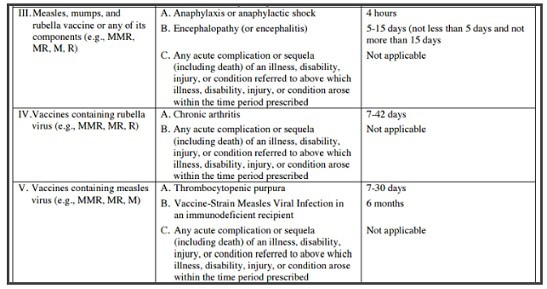
Combined measles, mumps, and rubella vaccine MMR was licensed April 22, 1971. The MMR vaccine has been controversial as it has been anecdotally linked to autism spectrum disorders in children. A Pace Environmental Law Review report “found eighty-three cases of autism among those compensated for vaccine-induced brain damage” by the US Vaccine Injury Compensation Program. Some of these 83 cases were from the MMR vaccine injuries. Dr. William Thompson’s 2014 statement admits that CDC researchers “omitted statistically significant information” … “and the final study protocol was not followed” in a 2004 study on the MMR vaccine’s relation to autism. The vaccine injury table below lists MMR adverse events, and when you can file a claim for injury.
The article below is on vaccinomics, personalized vaccinology. The main author is Gregory A. Poland, MD distinguished investigator of the Mayo Clinic, Director, Mayo Vaccine Research Group, Mayo Clinic College of Medicine Rochester, MN, Editor-in-Chief, Vaccine. Dr. Poland et al question the “one size fits all” vaccine approach:
Trends affecting the future of vaccine development and delivery: The role of demographics, regulatory science, the anti-vaccine movement, and vaccinomics (full text) Vaccine. 2009 May 26; 27(25-26): 3240–3244.
“As a result we attempt to deliver a series of vaccines to every living human on earth but it has been a ‘one size fits all’ approach, or population-level public health approach. In view of the advances in individualized medicine, we need to ask the question ‘is such an approach informed by the new science’? For example, currently a 1-year-old child and a 40-year-old 120 kg construction worker get the same dose of MMR vaccine.”
Every vaccine on the schedule has studies that show vaccine failure. In this study the failure in the rubella component of the vaccine can have dire consequences.
Genetic basis for variation of vaccine response: our studies with rubella vaccine (full text) Paediatrics and Child Health via NCBI December 2009 Volume 19, Supplement 2, Pages S156–S159
“As implied, there is a measurable primary and secondary failure rate. In one outbreak, 9.8% of all those vaccinated who had been 5 years before developed reinfection. In a resurgence of congenital rubella syndrome in California in 1990, 43% of cases of congenital rubella syndrome occurred among mothers with a history of rubella vaccination. In addition, reinfection clearly risks contagions. Those with subprotective levels of antibodies can become reinfected, this reinfection resulting in viraemia and subclinical infection. The shedding spreads the disease as well as the risk for congenital rubella syndrome. Finally, the vaccine causes both arthralgias and arthritis in 10–40% of susceptible females. Of note, some develop persistent joint reactions.”
When combining varicella with the measles, mumps, and rubella (ProQuad) there are more associated seizures.
Measles-Mumps-Rubella-Varicella Combination Vaccine and the Risk of Febrile Seizures Pediatrics July 2010, Volume 126 / Issue 1
“Vaccination with MMRV results in 1 additional febrile seizure for every 2300 doses given instead of separate MMR + varicella vaccines. Providers who recommend MMRV should communicate to parents that it increases the risk of fever and seizure over that already associated with measles-containing vaccines.”
VSD is the vaccine safety database, and is not available to the public, unlike VAERS. Most of the epidemiological studies use this database. It is very hard for physicians to access it.
Update: Recommendations from the Advisory Committee on Immunization Practices (ACIP) Regarding Administration of Combination MMRV Vaccine (full text) CDC MMWR Weekly March 14, 2008 / 57(10);258-260
“At its February 27, 2008, meeting, ACIP considered the preliminary results from the VSD and Merck studies, which suggested an increased risk for febrile seizures after the first dose of MMRV vaccine. Given the availability of alternative options for vaccination against measles, mumps, rubella, and varicella and the limited supply of MMRV vaccine, ACIP voted to change the preference language for MMRV vaccine to read as follows: “Combination MMRV vaccine is approved for use among healthy children aged 12 months–12 years.”
Questions remain regarding genetic and gender differences in immune responses:
Twin studies of immunogenicity — determining the genetic contribution to vaccine failure Vaccine. Volume 19, Issues 17–19, 21 March 2001, Pages 2434–2439
“Conclusion: Our data support the concept that genetic influences play a substantial role in the variation of antibody levels following immunization against measles and, to a lesser extent, mumps and rubella.”
Epidemic of Mumps among Vaccinated Persons, the Netherlands, 2009–2012 (full text) Emerging Infectious Diseases Volume 20, Number 4—April 2014
“The reasons for male predominance are unclear, but significantly higher mumps antibody titers in female than in male persons have been demonstrated); this finding, in turn, may be linked to gender-associated genetic differences in immune response.”
A very important 2001 study showed that the MMR vaccine can cause increased IgE. Could this IgE “class switching” caused by the MMR and other live virus vaccines be responsible for the increase in allergic disease?
Infection of Human B Lymphocytes with MMR Vaccine Induces IgE Class Switching Clinical Immunology Volume 100, Issue 3, September 2001, Pages 355-361
“Since many viral vaccines are live viruses, we speculated that live virus vaccines may also induce IgE class switching in human B cells. To examine this possibility, we selected the commonly used live attenuated measles mumps rubella (MMR) vaccine. Here, we show that infection of a human IgM+ B cell line with MMR resulted in the expression of germline epsilon transcript. In addition, infection of freshly prepared human PBLs with this vaccine resulted in the expression of mature IgE mRNA transcript. Our data suggest that a potential side effect of vaccination with live attenuated viruses may be an increase in the expression of IgE.“
Varicella Vaccine
Varicella vaccine is scheduled for infants 12 to 15 months with a booster at 4 to 6 years. Varivax, was licensed in 1995 and the MMR-V ProQuad was licensed in 2005.
A 2003 study from the UK questioned the cost-effectiveness and safety of universal varicella vaccination, and UK has not implemented universal vaccination to date, stating on their website, “There’s a worry that introducing chickenpox vaccination for all children could increase the risk of chickenpox and shingles in adults.”
Varicella vaccination in England and Wales: cost-utility analysis Archives of Disease in Childhood. 2003 Oct; 88(10): 862–869.
“Mathematical modelling based on these results predicts that, by reducing circulating VZV, universal varicella vaccination will lead to a significant increase in zoster, which can last up to 50 years. These findings support a re-evaluation of varicella vaccination, taking into consideration its impact on zoster. Here, we estimate, for the first time, the cost-utility varicella vaccination, taking account of the impact of varicella vaccination on all VZV associated disease.”… Conclusion Routine infant varicella vaccination is unlikely to be cost-effective and may produce an increase in overall morbidity. Adolescent vaccination is the safest and most cost-effective strategy, but has the least overall impact on varicella.”
There are concerns regarding the shedding of the vaccine virus:
Transmission of varicella-vaccine virus from a healthy 12-month-old child to his pregnant mother The Journal of Pediatrics Volume 131, Issue 1, July 1997, Pages 151–154
“A 12-month-old healthy boy had approximately 30 vesicular skin lesions 24 days after receiving varicella vaccine. Sixteen days later his pregnant mother had 100 lesions. Varicella-vaccine virus was identified by polymerase chain reaction in the vesicular lesions of the mother. After an elective abortion, no virus was detected in the fetal tissue.”
VAERS reports about serious side effects after the second dose of varicella vaccine are detailed here:
Safety of Second-Dose Single-Antigen Varicella Vaccine Pediatrics. 2017 Mar;139(3) 2016-2536. Epub 2017 Feb 7.
“We identified 14,641 Vaccine Adverse Event Reporting System reports after second-dose varicella vaccination, with 494 (3%) classified as serious. The most common AEs among children aged 4 to 6 years were pyrexia (fever) (31%) and headache (28%) and for children aged 7 to 18 years, was vomiting (27%). Serious reports of selected AEs included anaphylaxis (83), meningitis (5), encephalitis (16), cellulitis (52), varicella (6), herpes zoster (6), and deaths (7). One immunosuppressed adolescent was reported with vaccine-strain herpes zoster.”
An alarming 2-fold increased risk of seizure or febrile seizure after the MMR-V vaccine was reported, but more studies were needed “to confirm the findings.”
Risk of febrile seizure after measles–mumps–rubella–varicella vaccine: A systematic review and meta-analysis Vaccine Volume 33, Issue 31, 17 July 2015, Pages 3636–3649
“…an approximately 2-fold increase in risk of seizure or febrile seizure during 7–10 days or 5–12 days after MMRV vaccination was found among children aged 10–24 months, although the highest incidence of seizure was still lower than 2.95%. … Conclusion: First MMRV vaccine dose in children aged 10–24 months was associated with an elevated risk of seizure or febrile seizure. Further post-marketing restudies based on more rigorous study design are needed to confirm the findings.”
Vaccine Failure and maternal antibody interference:
Primary Vaccine Failure after 1 Dose of Varicella Vaccine in Healthy Children (full text) The Journal of Infectious Diseases (2008) 197 (7): 944-949.
“Primary vaccine failure in just 10% of vaccinees after a single dose could result in progressive accumulation of susceptible individuals over time and lead to an increased incidence of varicella in young adults. Such an increment is potentially dangerous. Approximately 4 million infants are vaccinated annually in the United States. A primary vaccine failure rate of 10% would thus lead to 400,000 vaccinated but susceptible infants every year. Within 5 years, there would be 2 million vaccinated but susceptible individuals. The present findings therefore strongly support the use of a second dose of vaccine for all children without a history of disease.”
Younger Age at Vaccination May Increase Risk of Varicella Vaccine Failure (full text) The Journal of Infectious Diseases (2002) 186 (1): 102-105.
“We found a trend toward increased risk of breakthrough disease with younger age at vaccination. Children vaccinated at <14 months of age had a 3 times higher risk of breakthrough than children vaccinated at ⩾14 months of age. In part because of small numbers, this finding did not achieve statistical significance. Data from a prelicensure trial of varicella vaccine indicated that infants and young children may have maternal antibody to varicella after their first birthday. [11] White et al. [11] reported that 47% of children at age 12 months had detectable antibody; by age 16 months, only 4% of children had detectable antibodies.”
Hepatitis A Vaccine
Note: Hepatitis A is required in 21 states (Immunization Action Coalition)
The Hepatitis A vaccine was introduced in 1995. The first recommendations were for children at risk. Hepatitis A vaccine is manufactured with human fetal cell lines. The following is a study explaining how DNA from fetal cells could cause autistic disorders. In those with mitochondrial disease the odds of antibody failure and adverse events are increased.
Genetic Evidence for Elevated Pathogenicity of Mitochondrial DNA Heteroplasmy in Autism Spectrum PLOS Genetics Published: October 28, 2016
“Although ASD is traditionally described as a developmental disorder of the central nervous system, emerging evidence suggests that systemic physiological abnormalities, including dysregulated inflammation and immune system, elevated oxidative stress, and mitochondrial dysfunction, are present in peripheral tissues as well as in brains of autistic patients.”
Vaccines, biotechnology and their connection with induced abortion. Cuad. Bioét. XIX, 2008/2ª
“Diploid cells (WI-38, MRC-5) vaccines have their origin in induced abortions. Among these vaccines we find the following: rubella, measles, mumps, rabies, polio, smallpox, hepatitis A, chickenpox, and herpes zoster. Nowadays, other abortion tainted vaccines cultivated on transformed cells (293, PER.C6) are in the pipeline: flu, Respiratory Syncytial and parainfluenza viruses, HIV, West Nile virus, Ebola, Marburg and Lassa, hepatitis B and C, foot and mouth disease, Japanese encephalitis, dengue, tuberculosis, anthrax, plague, tetanus and malaria.”
Impact of environmental factors on the prevalence of autistic disorder after 1979 The Journal of Public Health and Epidemiology Vol. 6(9), pp. 271-286, September 2014
“Varicella and Hepatitis A immunization coverage was significantly correlated to autistic disorder cases. R software was used to calculate change points. Autistic disorder change points years are coincident with introduction of vaccines manufactured using human fetal cell lines, containing fetal and retroviral contaminants, into childhood vaccine regimens. This pattern was repeated in the US, UK, Western Australia and Denmark. Thus, rising autistic disorder prevalence is directly related to vaccines manufactured utilizing human fetal cells.”
Another study on autoimmunity stated “Clinical evaluation of potential autoimmune side effects and the establishment of appropriate laboratory test protocols are crucial.”
Autoimmunity and Hepatitis A Vaccine in Children (pdf) Journal of Investigational Allergology and Clinical Immunology 2011; Vol. 21(5): 389-393
“We found transient ANA positivity in 25% of children after hepatitis A vaccination; just 2 of these children remained positive but there was no evidence of autoimmune disease. However, other factors such as ethnicity and environment may influence the appearance of autoantibodies following vaccination. In conclusion, a larger study is required to examine the relationship between hepatitis A vaccination and autoimmunity development. Clinical evaluation of potential autoimmune side effects and the establishment of appropriate laboratory test protocols are crucial.”
Studies reporting adverse events following hepatitis A vaccine:
Possible Association of Guillain-Barre Syndrome and Hepatitis A Vaccination (full text free registration) Pediatric Infectious Disease Journal June 2004 – Volume 23 – Issue 6 – pp 586-588 (free registration) through Medscape
“A 1 1/2-year-old previously healthy child was hospitalized for progressive weakness for 5 days. Ten days before admission, he received the first dose of the hepatitis A vaccine (HAVRIX 720). Five days later, he refused to walk, was weak and anorexic and had a low grade fever. No history of febrile illness was reported during the previous month.”…”In our patient, the association of Guillain-Barré syndrome with hepatitis A vaccine is supported by temporal proximity of the vaccination with the onset of symptoms, lack of other precipitating factors and the immune-mediated nature of the manifestation.”
Statistical and Ontological Analysis of Adverse Events Associated with Monovalent and Combination Vaccines against Hepatitis A and B Diseases (full text) Scientific Reports – Nature 2016; 6: 34318. Published online 2016 Oct 3
“Table 1 Havrix-specific adverse events, the AEs included in pregnancy, neonatal or perinatal disorder (e.g., abortion spontaneous and unintended pregnancy) were relatively frequent AEs.”
HAVRIX (Hepatitis A Vaccine) Suspension for Intramuscular Injection Initial U.S. Approval – Package Insert – FDA www.fda.gov
“Among subjects who received HAVRIX concomitantly with other childhood vaccines, 0.9% (8/909) reported a serious adverse event. In these 4 studies, there were 4 reports of seizure within 31 days post-vaccination these occurred 2, 9, and 27 days following the first dose of HAVRIX administered alone and 12 days following the second dose of HAVRIX. In 1 subject who received INFANRIX® (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) and Hib conjugate vaccine followed by HAVRIX 6 weeks later, bronchial hyperreactivity and respiratory distress were reported on the day of administration of HAVRIX alone.”
In addition to reports in clinical trials, worldwide voluntary reports of adverse events received for HAVRIX since market introduction of this vaccine are listed below. This list includes serious adverse events or events which have a suspected causal connection to components of HAVRIX or other vaccines or drugs.
Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
- Postmarketing Experience
- Infections and Infestations
- Rhinitis
- Blood and Lymphatic System Disorders
- Thrombocytopenia
- Immune System Disorders
- Anaphylactic reaction, anaphylactoid reaction, serum sickness–like syndrome.
- Nervous System Disorders
- Convulsion, dizziness, encephalopathy, Guillain-Barré syndrome, hypoesthesia, multiple sclerosis, myelitis, neuropathy, paresthesia, somnolence, syncope
- Vascular Disorders Vasculitis.
- Respiratory, Thoracic, and Mediastinal Disorders
- Dyspnea.
- Hepatobiliary Disorders
- Hepatitis, jaundice.
- Skin and Subcutaneous Tissue Disorders
- Angioedema, erythema multiforme, hyperhidrosis
- Congenital, Familial, and Genetic Disorders, Congenital anomaly.
- Musculoskeletal and Connective Tissue Disorders, Musculoskeletal stiffness.
- General Disorders and Administration Site Conditions
- Chills, influenza-like symptoms, injection site reaction, local swelling.”
Existing research has not tested entire schedule
A 2013 Institute of Medicine report titled The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies states:
“In summary, few studies have comprehensively assessed the association between the entire immunization schedule or variations in the overall schedule and categories of health outcomes, and no study has directly examined health outcomes and stakeholder concerns in precisely the way that the committee was charged to address in its statement of task. No studies have compared the differences in health outcomes that some stakeholders questioned between entirely unimmunized populations of children and fully immunized children. Experts who addressed the committee pointed not to a body of evidence that had been overlooked but rather to the fact that existing research has not been designed to test the entire immunization schedule. The committee believes that although the available evidence is reassuring, studies designed to examine the long-term effects of the cumulative number of vaccines or other aspects of the immunization schedule have not been conducted.”
There are over 19,000 serious adverse events in children under three according to VAERS (Vaccine Adverse Event Reporting System). These VAERS reports are a “small fraction” of the adverse events that occurred due to significant underreporting. An untested infant vaccination schedule with tens of thousands of adverse events reported is unacceptable. A moratorium on all vaccine mandates should be put in place immediately until this situation is rectified.
© 2017 Fearless Parent
Editor’s note: This is the fourth post in a series as reading background for The Truth About Vaccines docuseries. We scoured the scientific literature to understand the impacts on the infant immune system. This new white paper summarizes over 60 peer-reviewed (PubMed) studies on the 14 infant vaccines. The authors thank Louise Kuo Habakus for helping to make this paper possible. The entirety of this post is copyright protected. If you wish to share, please obtain prior written permission with details for partial use and link back for attribution. Thank you.
 Cindy Stolten started researching health issues affecting her first child in 1998, before the birth of her second child. Read about her family’s story here. Cindy is a board member and the website content manager for Vaccination News; administrative director for the Vaccine Research Library; and regional co-chair for PAMFA, the Pennsylvania Medical Freedom Alliance.
Cindy Stolten started researching health issues affecting her first child in 1998, before the birth of her second child. Read about her family’s story here. Cindy is a board member and the website content manager for Vaccination News; administrative director for the Vaccine Research Library; and regional co-chair for PAMFA, the Pennsylvania Medical Freedom Alliance.

Rita Hoffman began investigating the vaccine link to food anaphylaxis after her youngest child developed the immune system malfunction over 20 years ago. Her website is www.deadlyallergy.com. She is a board member of Vaccine Choice Canada and Vaccination News and has done administrative work at the Vaccine Research Library.
Photo credit: Adobe Stock #18405132











Thanks for the information! I have long ago arrived at the conclusion that vaccination is an organised criminal enterprise dressed up as disease prevention by means of junk science.
If we lived in a halfway sane society, vaccination would have been abolished a long time ago.
Kudos, and thank you for doing all this work. Amazing compilation.
Is there a schedule that you would recommend ?
Also, how do you feel about vaccinating older kids?
Mine are 12 and 8 and have only had one of each type of vaccine
Bob, I’d recommend a book by pediatrician Dr. Paul Thomas The Vaccine-Friendly Plan.
https://www.amazon.com/Vaccine-Friendly-Plan-Effective-Health-Pregnancy/dp/1101884231
Vaccines & Autism w/ Paul Thomas, MD (video)
https://www.youtube.com/watch?v=V7XdiX_BskA